Tokyo-based Terumo Corporation has agreed to acquire OrganOx, a University of Oxford spin-out specialising in automated organ preservation technology, for approximately $1.5 billion (£1.1 billion), marking the largest acquisition of an Oxford University spin-out to date.
The deal, announced on 25 August 2025, will see OrganOx become a wholly owned subsidiary of Terumo, a global leader in medical devices and blood management technologies.
Founded in 2008 by Professor Constantin Coussios and Professor Peter Friend from the University of Oxford, OrganOx emerged from research conducted at the Institute of Biomedical Engineering and the Nuffield Department of Surgical Sciences.
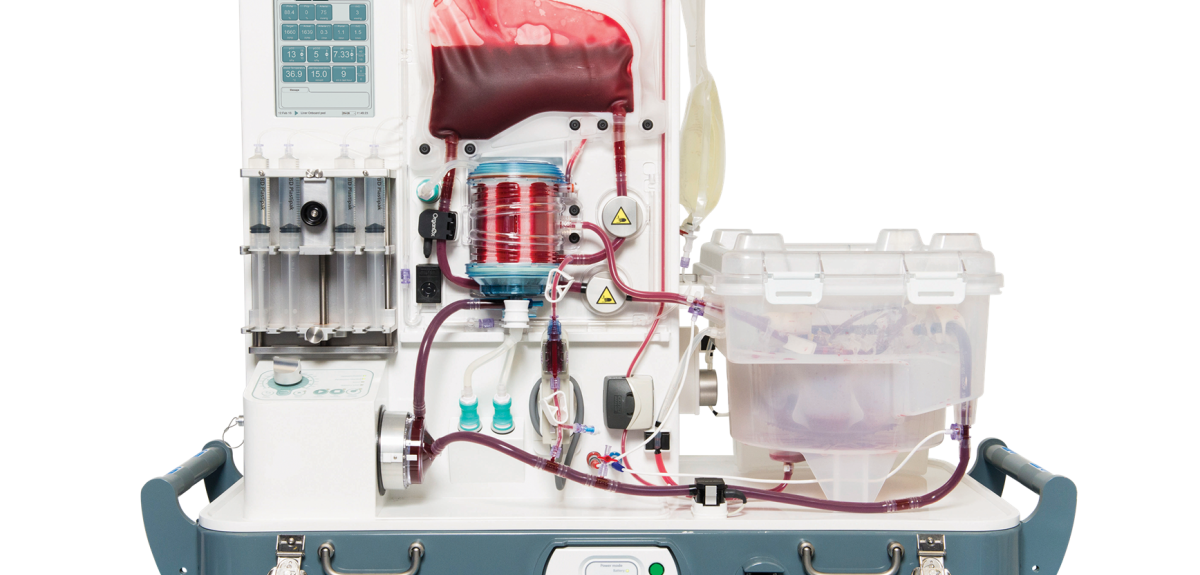
The company pioneered the Metra® system, a normothermic machine perfusion (NMP) platform that maintains donor livers in near-physiological conditions by circulating oxygenated, nutrient-rich blood at body temperature.
Unlike traditional cold storage, which halts metabolic activity, NMP allows organs to remain functional during transport, enabling viability assessment and repair before transplantation.
According to clinical data cited by the company and reported in peer-reviewed studies, the Metra® system has been used in over 6,000 liver transplants worldwide and is associated with improved graft survival and reduced rates of early allograft dysfunction.
The acquisition follows a period of rapid financial growth for OrganOx. In February 2025, the company raised $142 million in equity financing led by the UK’s Business Growth Fund (BGF) and US-based HealthQuest Capital.
A second private placement in May brought total capital raised that year to $160 million. OrganOx achieved profitability in 2024, reporting £55 million in revenue and £7.2 million in operating profit—key milestones that enhanced its attractiveness to strategic buyers.
BGF, which first invested in 2019 and became the largest shareholder, is expected to receive approximately £175 million from the sale, representing a tenfold return on its initial investment, according to figures reported by The Financial Times and The Times.
For Terumo, the acquisition represents a strategic expansion into the organ transplantation space, a niche but high-potential segment within the broader med-tech industry. While Terumo has long been active in cardiovascular and blood component technologies, it has not previously held a presence in organ preservation.
The Metra® system is already approved for clinical use in multiple jurisdictions, including the United States (FDA-cleared), the European Union (CE-marked), Canada, and Australia, offering Terumo an established commercial platform with proven clinical adoption.
With operations in over 160 countries and a workforce of more than 30,000 employees, Terumo is well-positioned to accelerate the global rollout of OrganOx’s technology, particularly in emerging markets where transplant infrastructure is developing.
In recent years, several high-potential spin-outs have been acquired by foreign firms before reaching IPO stage. The 2025 sale of Oxford Ionics, a quantum computing company, to US-based IonQ for $1.1 billion followed a similar trajectory, reinforcing concerns about the UK’s ability to retain ownership of its most promising deep-tech ventures.
According to data from the Office for Life Sciences, foreign buyers accounted for nearly 60% of UK life sciences exits between 2020 and 2024, a figure that has sparked debate among policymakers about the need for a national investment strategy to support scaling.
From a medical standpoint, normothermic machine perfusion is increasingly viewed as a transformative tool in transplantation. By extending the viability window of donor organs and enabling functional assessment, NMP systems like Metra® can reduce organ discard rates, currently estimated at 30–40% for livers in some regions, and expand the pool of usable organs, including those from older or marginal donors.
Ongoing clinical trials suggest NMP may also facilitate organ repair and regeneration, opening pathways for future regenerative therapies.
OrganOx continues to invest in next-generation applications. The company is developing a kidney-specific NMP device, with commercial launch anticipated around 2030. Additionally, it has entered a research collaboration with eGenesis, a US-based biotech firm advancing xenotransplantation through gene-edited pig organs.
Early-stage trials are exploring the use of Metra® to perfuse genetically modified pig livers, a development that could address long-term organ shortages if regulatory and immunological hurdles are overcome.